The Project
For the development of a risk-based screening strategy, AFFECT-EU’s methodology will consist of
- A confirmatory part using well-powered, large-scale clinical trial information as it becomes available to define the currently best AF screening strategy for implementation. We will also integrate results from multiple smaller trials/studies across Europe.
- An exploratory part with a broader search for markers for risk stratification in the population to further improve AF screening. Since Nt-proBNP is currently the most promising biomarker for risk refinement, we will measure it in all studies with available blood samples. In an orchestrated approach, we will thus combine the abundance of locally existing data from high quality cohorts and trials with the aim to maximise access and jointly explore data to achieve exclusively robust results during the funding period.
- A patient level meta-analysis, in which results of the conceptually similar trials will be aggregated as they become available to increase statistical power and provide robust point estimates.
- Cost-benefit analyses are a mandatory part that accompanies and guides the development and refinement of the risk-based screening algorithm and also serves to evaluate inequalities. Health economics and cost-benefit analyses,
- Dissemination, exploitation, commincation and implementation at scale, e.g. EHRA (ESC), AF-SCREEN) and include multifaceted stakeholder’s inputs
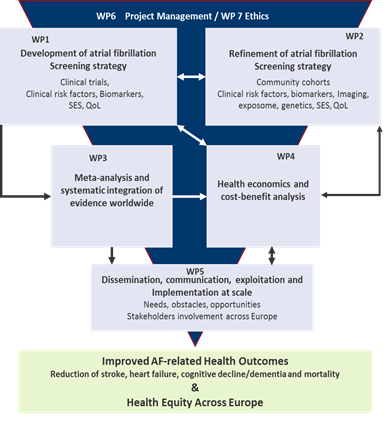
Integrated research and implementation work-flow for the development of a targeted, risk-based screening intervention in the European community.
